Adsorption Isotherm | Surface Chemistry | Chemistry 12th
Автор: Aman Singh
Загружено: 2020-08-25
Просмотров: 289
For other videos of this chapter click below link :
• Chemistry 12th || Chapter 5 : Surface Chem...
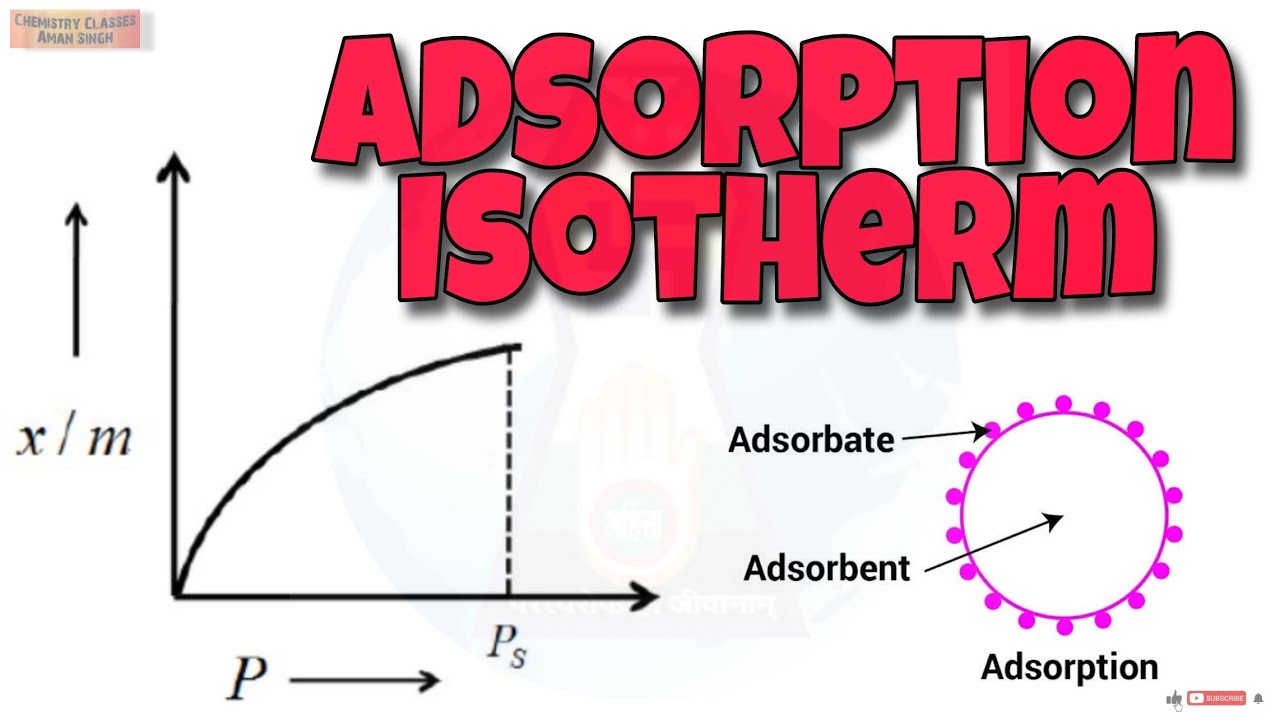
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm. Freundlich adsorption isotherm: Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship is generally represented in the form of a curve where mass of the gas adsorbed per gram of the adsorbent is plotted against pressure . These curves indicate that at a fixed pressure, there is a decrease in physical adsorption with increase in temperature. These curves always seem to approach saturation at high pressure. The experimental isotherms always seem to approach saturation at high pressure. This cannot be explained by Freundlich isotherm. Thus, it fails at high pressure.
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:


















