Lec 03: Variable valency Oxidation states & Relative stability of oxidn States in transition metals
Автор: AK chemistry
Загружено: 2020-09-25
Просмотров: 17103
Thanks for your Love
Like share and subscribe.
This lecture explain why transition elements show variable vacancy, different oxidation states in transition metals and Relative stability of different oxidation states.
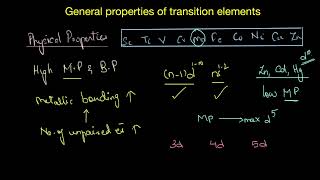
Lecture starts with the introduction to Valency and how electrons are filled and how to remove them from transition metals.
Lecture explain why the 4s electrons are removed first compared to 3d subshell.
Based on this concept we have different oxidation states possible for the transition elements depending upon their tendency to lose ns and (n-1)d electrons.
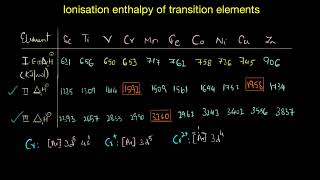
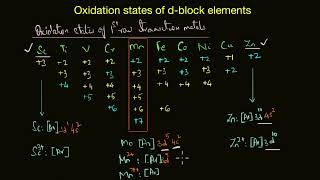
Lecture explain the various Oxidation states possible in transition elements by considering their electronic configuration.
Lecture also take the concept of which ligands can form bond with transition metals in their higher oxidation states while some metals can form complexes in their low oxidation i.e. unusual oxidation state for metals ( -ve, 0, low +ve oxidation state).
Lecture then explain the Relative stability of different oxidation states in transition metals i.e. which oxidation state is more stable in these complexes.
Lecture consider the case of Carbonyl ligand
#Variablevalency
#Valencu
#oxidationstates
#transtionelements
#Carbonylligand

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:












![8.2- d~Block elements , physical properties [Variable Oxidation States of transition elements ]](https://ricktube.ru/thumbnail/nxwnRJDHt2s/mqdefault.jpg)


![Magnetic Properties of transition elements [ spin & orbital contribution to magnetic moment ]](https://ricktube.ru/thumbnail/jduvWbIpeF0/mqdefault.jpg)
