5.2 | Diffusion | Effusion | Examples | Chemistry Class 9th
Автор: Al-Farooq Academy
Загружено: 2023-08-04
Просмотров: 5083
This Video Explains the Process of Diffusion & Effusion in gases, their examples & their differences.
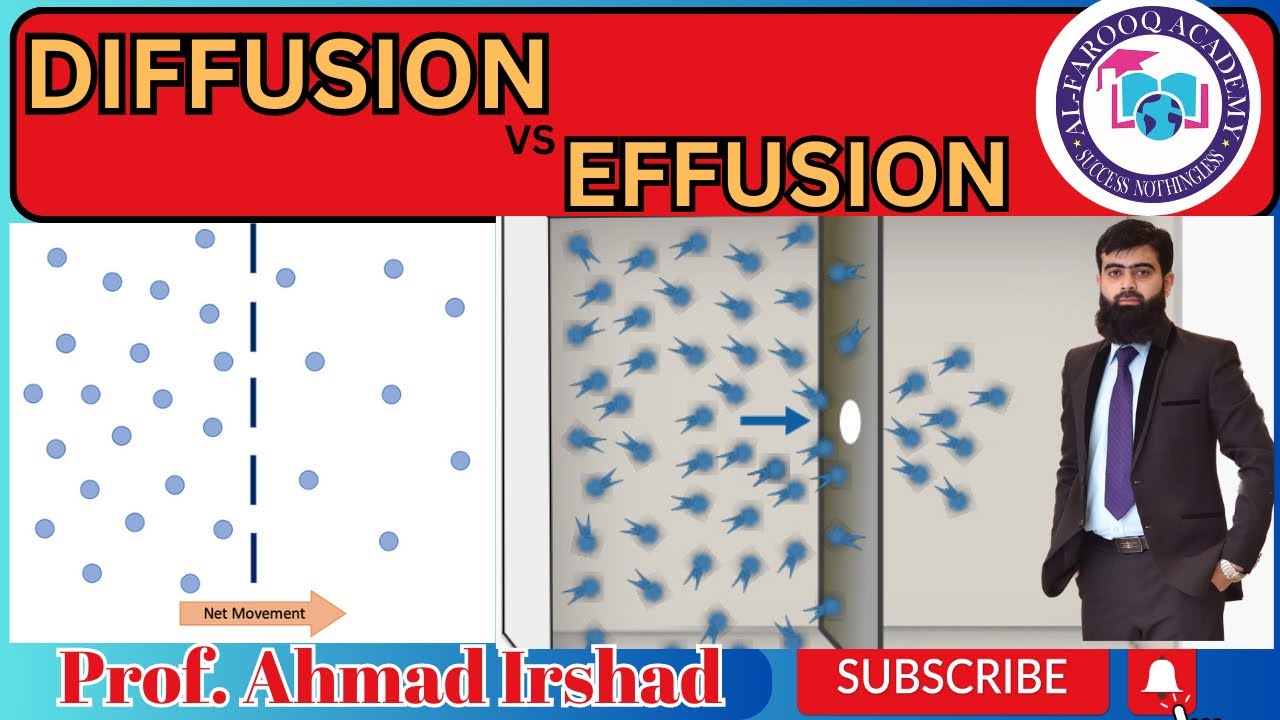
Diffusion is defined as spontaneous mixing up of molecules by random motion and collisions to form a homogeneous mixture. Rate of diffusion depends upon the molecular mass of the gases. Lighter gases diffuse rapidly than heavier ones. For example, H, diffuses four times faster than O, gas.
Effusion is escaping of gas molecules through a tiny hole into a space with lesser pressure. For example, when a tyre gets punctured, air effuses out. Effusion depends upon molecular masses, lighter gases effuse faster than heavier gases.
Follow us on:
FACEBOOK:
https://www.facebook.com/AlFarooq.Aca...
INSTAGRAM:
https://instagram.com/alfarooqacademy...
WHATSAPP: 03071070723
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:



















