PERIODIC TABLE (THE TRANSITION ELEMENTS)
Автор: 7activestudio
Загружено: 2013-10-23
Просмотров: 98508
Follow us: / 7activestudio
For more information:
www.7activestudio.com
[email protected]
Contact: +91- 9700061777, 040-66564777
7 Active Technology Solutions Pvt.Ltd. is an educational 3D digital content provider for K-12. We also customise the content as per your requirement for companies platform providers colleges etc . 7 Active driving force "The Joy of Happy Learning" -- is what makes difference from other digital content providers. We consider Student needs, Lecturer needs and College needs in designing the 3D & 2D Animated Video Lectures. We are carrying a huge 3D Digital Library ready to use.
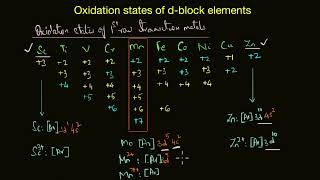
THE TRANSITION ELEMENTS d- BLOCK ELEMENTS. Position in the periodic table:In the modern periodic table, elements are classified into four blocks on the basis of electronic configuration. Elements in which differentiating electron enter d-orbitals in penultimate shell are called d-block elements.These elements are placed in between s and p-blocks in four series.They are 3d,4d,5d and 6d series. First three series of d-blocks are completely filled, with ten elements in each series and 6d-series is incomplete.3d-series elements are scandium z=21 to zincz=30, 4d series elements are yttriumz=39 to cadmiumz=48 and 5d-series elements are lanthanumz=57, hafniumz=72 to mercuryz=80.d-block consists of ten groups 3B, 4B, 5B, 6B, 7B, 1B and 2B.In a broader sense, d-block elements are called transition elements.This is because, d-block elements represent transition in properties from most electro positive s-block elements to least electropositive p-block elements.In transition elements, ultimate and penultimate elements to least electro positive p-block elements.In transition elements, ultimate and penultimate shells are in completely filed with electrons.Elements which have partially filled d-sub shells either in their elemental form or in any of their chemically significant oxidation state, are called transition elements.Thus 2B group elements Znzinc, Cdcadmium, and Hgmercury,are d-block elements but not transition elements, because they do not have in completely filled d-sub shellboth in elemental forms in divalent state. So, except2B group elements, remaining d-block elements are transition elements.All transition elements are d-block elements but all d-block elements are not transition elements.

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке: