Writing A Balanced Chemical Equation, Determining Limiting Reactant, and Amount of Product Produced
Автор: Atomic Answers
Загружено: 2025-10-25
Просмотров: 1747
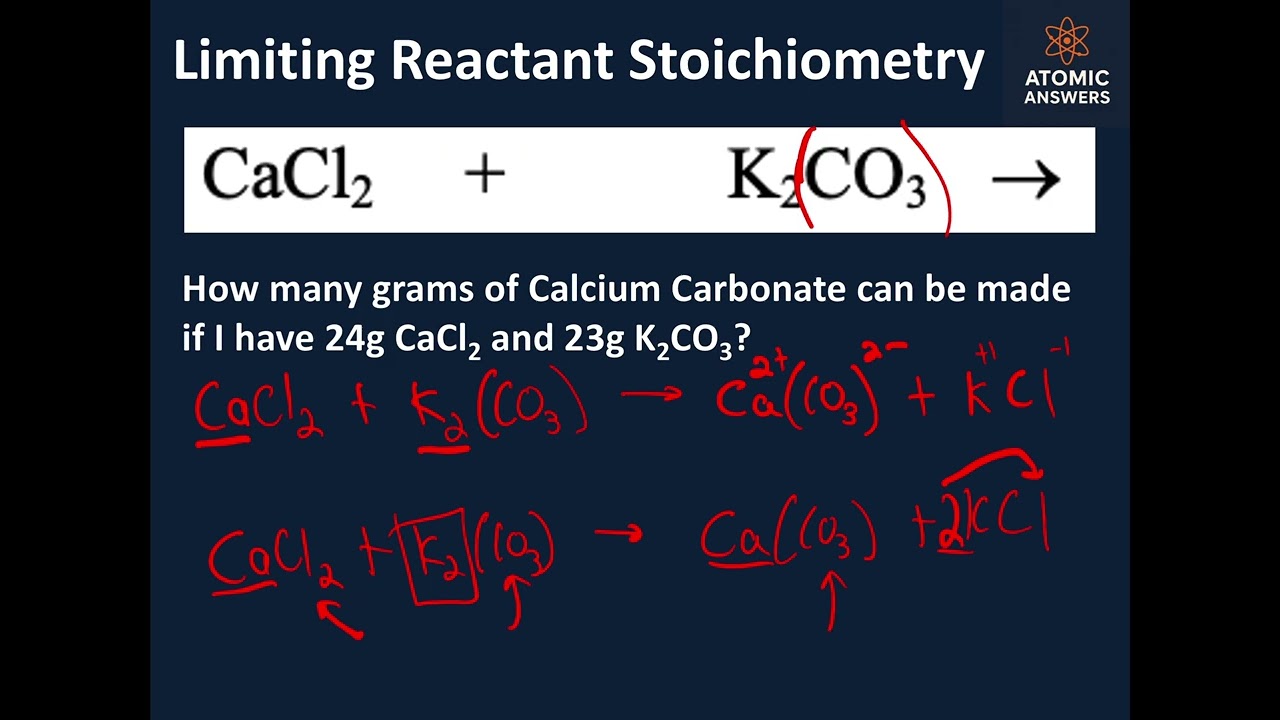
In this capstone stoichiometry problem, we start with only the reactants — no products are given! Step by step, we predict products, write and balance the chemical equation, determine the limiting reactant, and calculate how many grams of product can actually be produced.
This video pulls together everything you’ve learned in stoichiometry so far — perfect for test review or mastering the entire process from start to finish. If you’ve ever struggled to connect predicting products, balancing, and mole-to-gram conversions into one seamless problem, this video will make it all click.
You’ll learn how to:
Predict products from given reactants
Write and balance complex reactions correctly
Identify the limiting reactant with full reasoning
Use mole ratios to go from grams of reactant → grams of product
Apply all stoichiometric steps in a single integrated problem
Topics Covered:
Balancing | Limiting Reactant | Stoichiometry | Reaction Prediction | Mass-Mass Conversions | Chemistry Problem-Solving
#AtomicAnswers #ChemistryHelp #Stoichiometry #LimitingReactant #Chemistry
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:



















