Carbene | General Organic Chemistry | Jee Mains, Advance & BITSAT | NEET & AIIMS
Автор: IITian explains by Unacademy
Загружено: 2018-02-20
Просмотров: 78282
A carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R-(C:)-R' or R=C:. The term "carbene" may also refer to the specific compound H2C:, also called methylene, the parent hydride from which all other carbene compounds are formally derived. Carbenes are classified as either singlets or triplets depending upon their electronic structure. Most carbenes are very short lived, although persistent carbenes are known. One well studied carbene is Cl2C:, or dichlorocarbene, which can be generated in situ from chloroform and a strong base.
Singlet and triplet carbenes
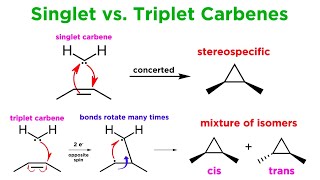
The two classes of carbenes are singlet and triplet carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory, the molecule adopts an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. They may be either linear or bent, i.e. sp or sp2 hybridized, respectively. Most carbenes have a nonlinear triplet ground state, except for those with nitrogen, oxygen, or sulfur atoms, and halides directly bonded to the divalent carbon.
Carbenes are called singlet or triplet depending on the electronic spins they possess. Triplet carbenes are paramagnetic and may be observed by electron spin resonance spectroscopy if they persist long enough. The total spin of singlet carbenes is zero while that of triplet carbenes is one (in units of [\hbar] ). Bond angles are 125-140° for triplet methylene and 102° for singlet methylene (as determined by EPR). Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.
For simple hydrocarbons, triplet carbenes usually have energies 8 kcal/mol (33 kJ/mol) lower than singlet carbenes (see also Hund's rule of maximum multiplicity), thus, in general, triplet is the more stable state (the ground state) and singlet is the excited state species.Substituents that can donate electron pairs may stabilize the singlet state by delocalizing the pair into an empty p-orbital. If the energy of the singlet state is sufficiently reduced it will actually become the ground state. No viable strategies exist for triplet stabilization. The carbene called 9-fluorenylidene has been shown to be a rapidly equilibrating mixture of singlet and triplet states with an approximately 1.1 kcal/mol (4.6 kJ/mol) energy difference.[3] It is, however, debatable whether diaryl carbenes such as the fluorene carbene are true carbenes because the electrons can delocalize to such an extent that they become in fact biradicals. In silico experiments suggest that triplet carbenes can be thermodynamically stabilized with electropositive heteroatoms such as in silyl and silyloxy carbenes, especially trifluorosilyl carbenes.
Reactivity
Carbene addition to alkenes
Singlet and triplet carbenes exhibit divergent reactivity. Singlet carbenes generally participate in cheletropic reactions as either electrophiles or nucleophiles. Singlet carbenes with unfilled p-orbital should be electrophilic. Triplet carbenes can be considered to be diradicals, and participate in stepwise radical additions. Triplet carbenes have to go through an intermediate with two unpaired electrons whereas singlet carbene can react in a single concerted step.
Due to these two modes of reactivity, reactions of singlet methylene are stereospecific whereas those of triplet methylene are stereoselective. This difference can be used to probe the nature of a carbene. For example, the reaction of methylene generated from photolysis of diazomethane with cis-2-butene or with trans-2-butene each give a single diastereomer of the 1,2-dimethylcyclopropane product: cis from cis and trans from trans, which proves that the methylene is a singlet. If the methylene were a triplet, one would not expect the product to depend upon the starting alkene geometry, but rather a nearly identical mixture in each case.
Reactivity of a particular carbene depends on the substituent groups. Their reactivity can be affected by metals. Some of the reactions carbenes can do are insertions into C-H bonds, skeletal rearrangements, and additions to double bonds. Carbenes can be classified as nucleophilic, electrophilic, or ambiphilic. For example, if a substituent is able to donate a pair of electrons, most likely carbene will not be electrophilic. Alkyl carbenes insert much more selectively than methylene, which does not differentiate between primary, secondary, and tertiary C-H bonds.
C—H insertion
Insertions are another common type of carbene reactions. The carbene basically interposes itself into an existing bond. The order of preference is commonly: 1. X–H bonds where X is not carbon 2. C–H bond 3. C–C bond. Insertions may or may not occur in single step.
For more, watch our lecture in details. We hope you will definitely enjoy it.
Thanks
Team IITian explains.

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке: