How to Determine aromaticity: The Cycloheptatrienyl Cation Explained (Huckel's rule)
Автор: Globealchemy Chemistry
Загружено: 2026-01-11
Просмотров: 7
@GlobealchemyChemistry #How to Determine Aromaticity: The Cycloheptatrienyl Cation Explained (Huckel's rule) #Tropylium cation #Cycloheptatrienyl cation #Huckel's Rule #Aromaticity #Jee #Neet #Organic Chemistry #LearnO Chemistry #IIT JEE #GOC #Class11 #class12
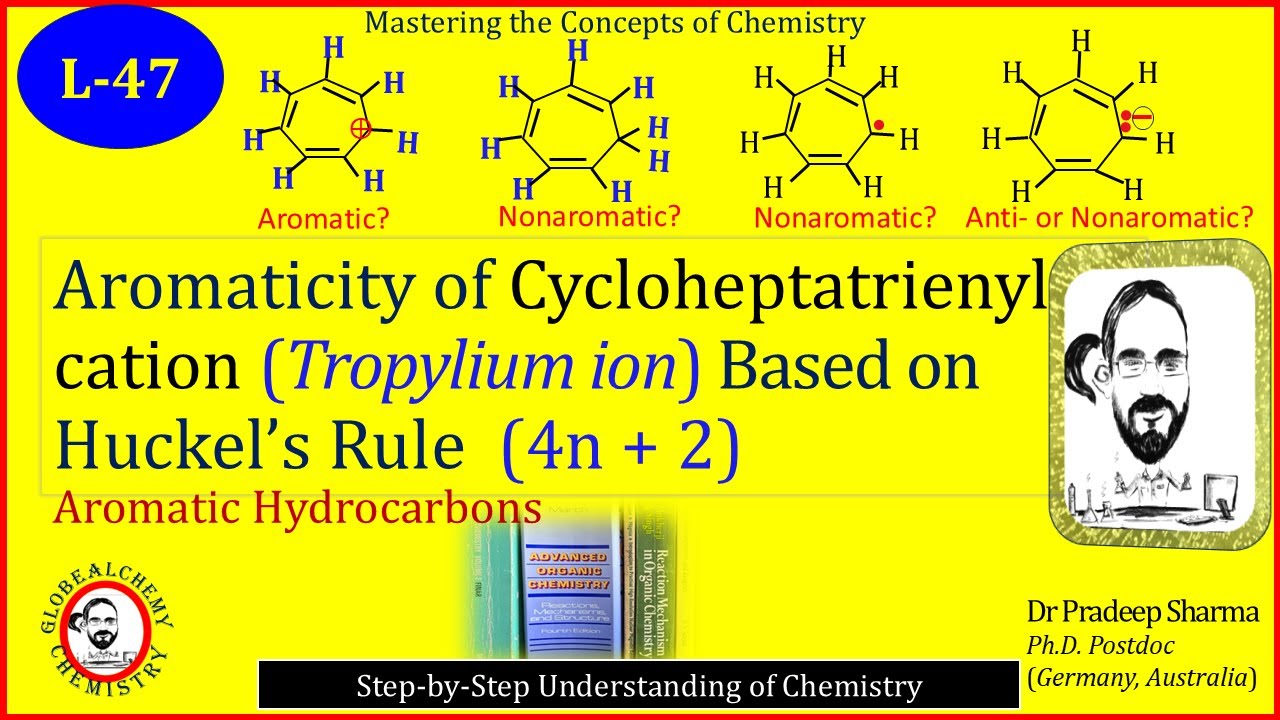
This lecture explains how to determine aromaticity using Hückel’s rule, with the cycloheptatrienyl cation (tropylium ion) as a key example. The structure, planarity, conjugation, and π-electron count of the cycloheptatrienyl cation are discussed to show why it satisfies the (4n + 2) rule and is aromatic. In addition, the cycloheptatrienyl anion and cycloheptatrienyl radical are also examined. Although their electron counts might suggest nonaromatic or antiaromatic character, their lack of continuous planarity due to unpaired and paired electrons and effective conjugation makes them nonaromatic rather than antiaromatic.
Please follow the following lectures on aromaticity, Huckel's rule
• Aromaticity | Stability of the Benzene Rin...
• Huckel's Rule and Aromaticity | 4n+2 Rule ...
• Aromaticity of Cyclopentadienyl Anion Base...
• Aromaticity of Cyclopropenyl Cation Based...
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:



















