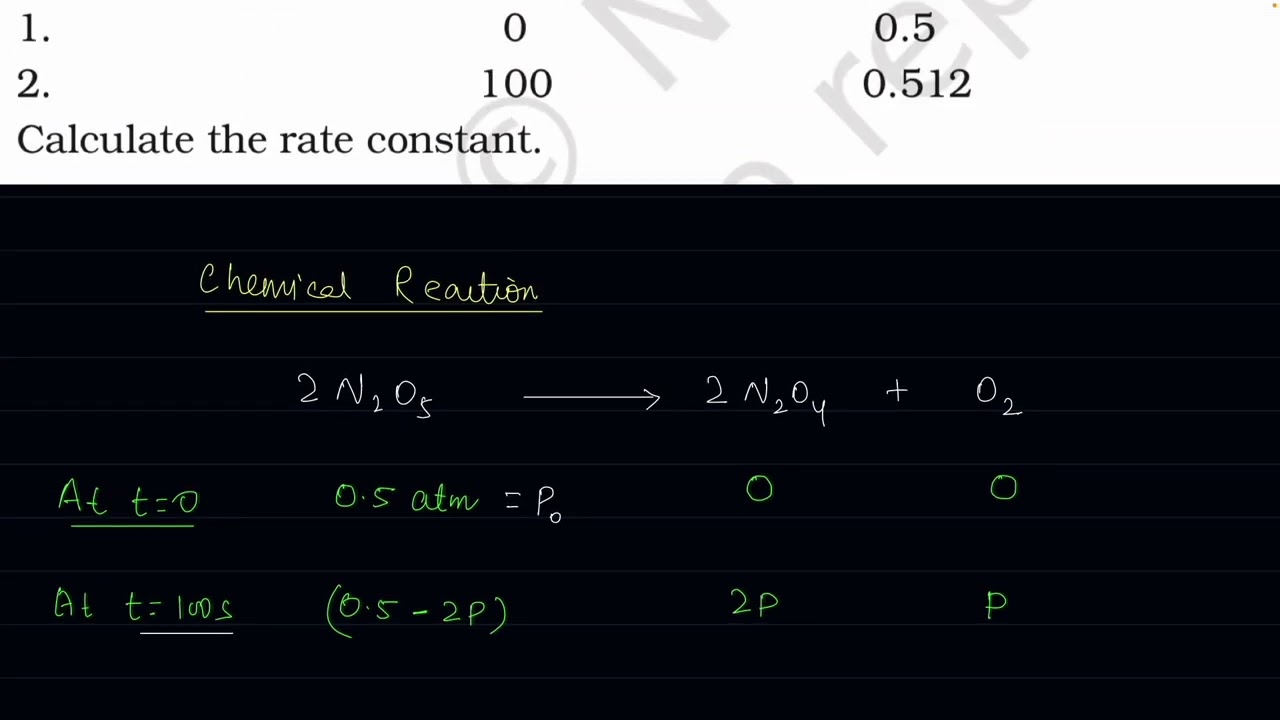
The following data were obtained during the first order thermal decomposition of N2O5 (g) at const
Автор: StudyDoubt
Загружено: 2025-07-24
Просмотров: 155
The following data were obtained during the first order thermal decomposition of N2O5 (g) at constant volume:
N205 --- NO2O4 + O2
Time/s Total Pressure/(atm)
1. 0 0.5
2. 100 0.512
Calculate the rate constant. @Study-Doubt
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:


















![For the reaction : 2A+B rarr A_(2)B the rate =k[A][B]^(2) with k=2.0xx10^(-6)mol^(-2)L^(2)s^(-1)...](https://image.4k-video.ru/id-video/4TPKzcCf7W4)
