Discoveries to Atomic Models | Thomson, Rutherford, and Millikan’s Work | Exam Essential Series
Автор: Biology Goal
Загружено: 2025-09-25
Просмотров: 50
Download the lecture notes free
Lecture Notes: https://drive.google.com/file/d/1m5zG...
Annotative notes: https://drive.google.com/file/d/19BLS...
Explore My Research Courses: https://www.researchgoal.com
✅ Get a certificate after completion
✅ Join a WhatsApp group with me for direct guidance
✅ Get mentorship, support, and tools to solve real research problems
It’s a great way to upskill, connect, and move your research forward.
Email: [email protected] Phone +91 7018393098
Exam Relevance
This topic is essential for learners across all levels—from high school to doctoral research. At the school and pre-university level, it underpins exams such as CBSE/ICSE Boards, JEE Main & Advanced, NEET in India, as well as SAT Chemistry, AP Chemistry, GCSEs, Cambridge O-Levels and A-Levels, IB SL/HL, and HSC exams internationally. At the undergraduate and postgraduate level, it is central to competitive exams like IIT-JAM, GATE, CSIR-UGC NET, DBT-RA, and JEST in India, alongside GRE Physics, MCAT, and other international graduate entrance tests. At the research and PhD level, atomic structure and experimental models form a core part of qualifying and comprehensive examinations worldwide.
SEO Keywords:
Structure of atom, atomic theory, X-rays discovery, radioactivity discovery, Thomson model of atom, Rutherford gold foil experiment, Rutherford nuclear model, Millikan oil drop experiment, discovery of electron charge, atomic structure explained, subatomic particles, nuclear atom, CSIR NET atomic structure, IIT JAM chemistry, NEET physics atoms, GRE physics atomic theory, AP chemistry atomic structure, A-level physics atom, DBT RA exam preparation, GATE physics chemistry, Research Goal education
1. Discovery of X-Rays and Radioactivity
Wilhelm Röentgen (1895):
• Produced when high-speed electrons strike a dense target.
• Not affected by electric or magnetic fields.
• Extremely penetrating (~0.1 nm wavelength).
• Later confirmed as electromagnetic radiation.
This discovery gave science a new tool to probe the unseen interior of matter.
Henri Becquerel (1896):
• α-rays: Heavy, positively charged (helium nuclei).
• β-rays: High-speed electrons.
• γ-rays: Neutral, highly penetrating radiation.
These findings exposed the complex internal architecture of atoms, showing they were far from indivisible.
________________________________________
2. Thomson’s Model of the Atom (1898)
Building on his discovery of the electron, J.J. Thomson proposed the plum pudding model:
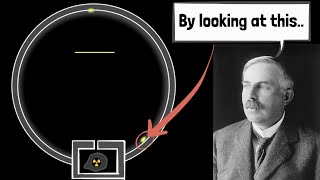
3. Rutherford’s α-Scattering Experiment (1909)
To test Thomson’s model, Rutherford (with Geiger and Marsden) bombarded thin gold foil with α-particles. The screen around it flashed when particles struck.
________________________________________
4. Rutherford’s Nuclear Model (1911)
• Atoms are mostly empty space.
• Positive charge and most mass are concentrated in a tiny nucleus.
• Electrons orbit the nucleus like planets around the sun.
Analogy: If the atom is a 5 km stadium, the nucleus is a cricket ball at the center.
________________________________________
5. Millikan’s Oil Drop Experiment (1909–1911)
Thomson had measured the charge-to-mass ratio (e/m) of the electron, but the absolute charge was unknown. Robert Millikan devised an ingenious method:
Closing Reflection
Together, these discoveries revolutionized atomic theory:
• Röentgen and Becquerel revealed the hidden world of radiation.
• Thomson proposed the first atomic model.
• Rutherford uncovered the nucleus.
• Millikan measured the quantized charge of the electron.
🔔 Don’t forget to like, share, and subscribe for more insightful videos on cell signaling and beyond!
WhatsApp group: https://chat.whatsapp.com/HTXQC1Ax2qf...
Twitter: / basic_series
Facebook Group: / 742235929758671
LinkedIn: / basicscienceseriesa54439208
Instagram: / basic_science_series
Support my work at https://www.patreon.com/user?u=37177596
Disclaimer: The information provided is for educational purposes only. The content of this channel should not be considered as medical advice of any kind. Use this information at your own risk. We hold no responsibility for any issue, concerns, or damage arising from the content of the video. Under no circumstances this channel be responsible or liable in any way for any content, including but not limited to, any errors or omissions in the content, any loss, any damage of any kind incurred as a result of any content communicated in this video, whether by this channel or a third party. In no event shall this channel be liable for any special indirect or consequential damages of any damages whatsoever resulting from the content of the channel.

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке: