Supplier and Internal Auditing
Автор: GlobalCompliance Panel
Загружено: 2016-05-27
Просмотров: 9063
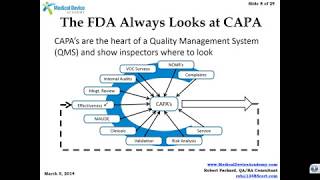
The FDA regulations contain a vast quantity of requirements, which govern tasks performed by your company's personnel every day.
Are there going to be noncompliance? You bet. Are you going to correct these noncompliances? Only if you identify them. To ensure substantial compliance, your company needs an effective audit program for both internal and external processes. To have an effective audit program, your company needs an effective audit team.
This Video will detail the best methods or training auditors, and include the best methods for performing internal audits as well as audits of suppliers and subcontractors.
For More Information Contact -
Organization: NetZealous BDA GlobalCompliancePanel
Website: http://www.globalcompliancepanel.com/
Email: [email protected]
Help us caption & translate this video!
http://amara.org/v/Japu/

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке: