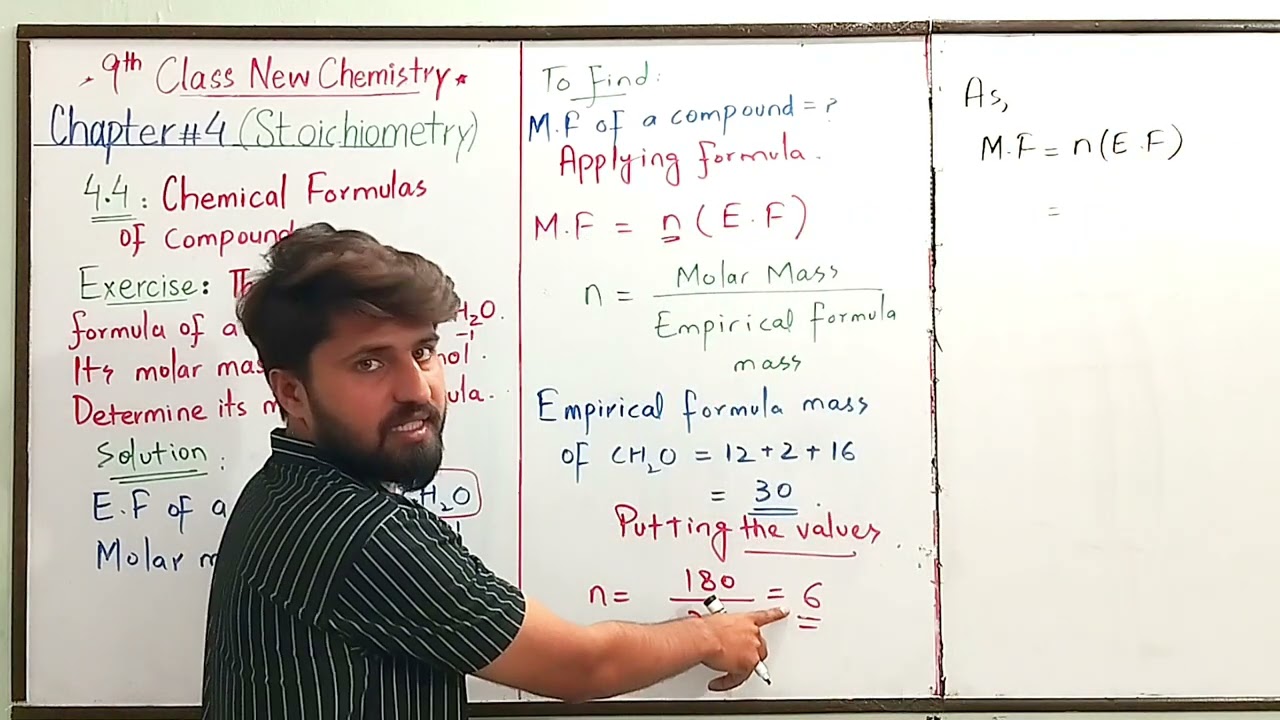
Empirical formula of a compound is CH2O.It's molar mass is 180g/mol.Determine it's molecular formula
Автор: Chemistry by Shoaib Javed
Загружено: 2025-06-05
Просмотров: 4480
#exercisequestions
#question 1:The Empirical formula of a compound is CH2O.It's molar mass is 180g/mol.Determine it's molecular formula.
#question 2:The Empirical formula of a compound is CH2O. It's molar mass is 60 g/mol. Determine it's molecular formula.
#solution of two exercise questions.
#how to calculate the molecular formula?
#how to calculate the molecular formula from Empirical formula?
#molarmass is given.
#molecularformula ?
#9thclasschemistry
#education
#newbook chemistry 9th Class
#punjabtextbookboard
#ptb
#nationalcurriculum
#class 9 chemistry
#chemistrybyShoaibjaved
#chapter4
#stoichiometry
#stoichiometric calculations.
#molecularformula of glucose and acetic acid.
#educationalvideo
#educationalcontent
#viralvideo
#chemistry by Shoaib Javed
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:



















