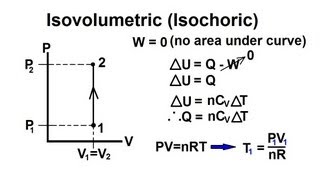
Isochoric/Isovolumetric (Constant Volume) Process on P-V Diagram
Автор: Elucyda
Загружено: 2020-08-30
Просмотров: 12328
A piston (containing an ideal gas) undergoing an isochoric/isovolumetric process is represented on the P-V diagram.
#Thermodynamics
#Isovolumetric
#KonstantinLakic

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке: