Free Radical Substitution (Ethane and bromine)
Автор: chemistNATE
Загружено: 4 дек. 2017 г.
Просмотров: 221 983 просмотра
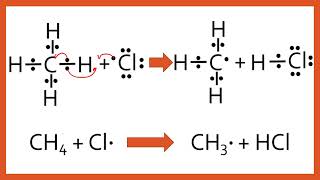
An alkane can get fluorinated/chlorinated/brominated by Free Radical Substitution.
Initiation: Break apart the Cl2 with UV light to make free radicals
Propagation: The Cl radical rips an H from the alkane, leaving it as an alkyl free radical. Then the alkyl free radical steals a Cl from another Cl2 molecule, leaving a Cl radical again, and this makes Cl radical a catalyst.
Termination: Two radicals combine to form a non-radical.

Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке: