Total Synthesis of Arcutinidine
Автор: The Cyclo Edition
Загружено: 2019-07-22
Просмотров: 4590
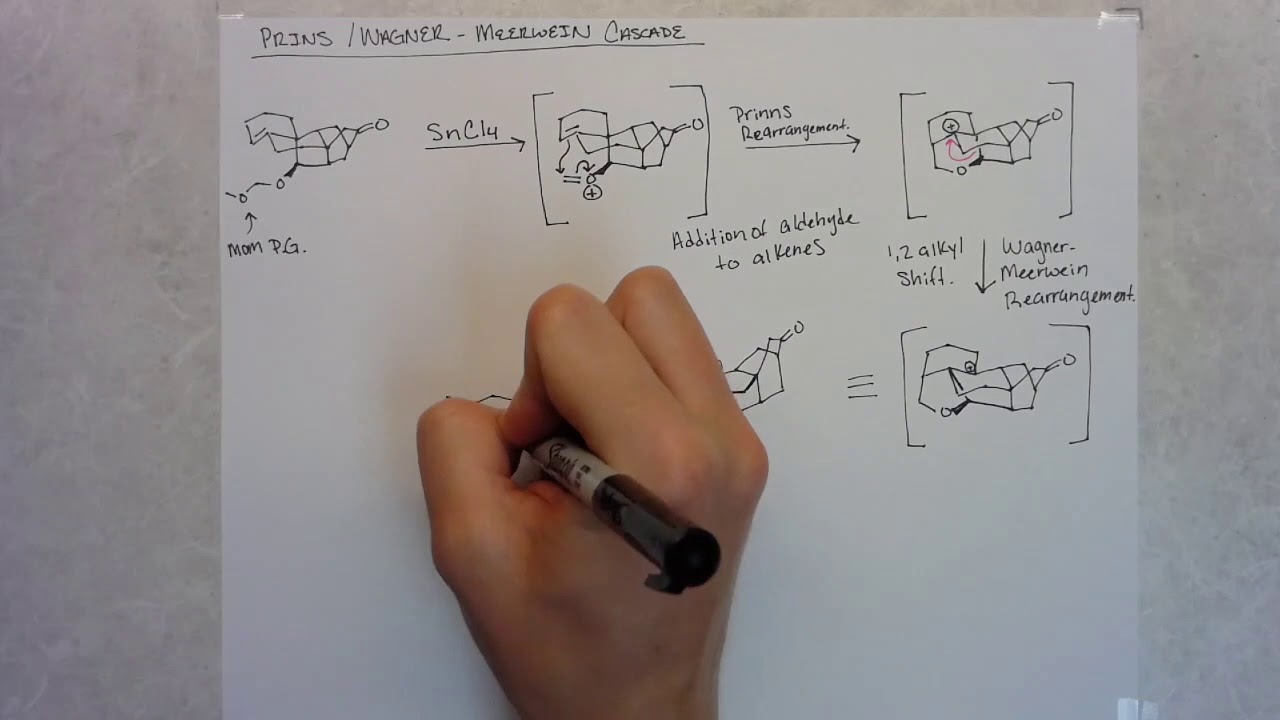
In this episode we analyze the total synthesis of arcutinidine developed by the Li lab and compare it to recent syntheses completed by the Sarpong and Qin labs.
DOI: 10.1021/jacs.9b05818
References:
Zhou, S.; Xia, K.; Leng, X.; Li, A. Asymmetric Total Synthesis of Arcutinidine, Arcutinine, and Arcutine. J. Am. Chem. Soc. 2019.
Owens, K. R.; McCowen, S.; Blackford, K. A.; Ueno, S.; Hirooka, Y.; Weber, M.; Sarpong, R. Total Synthesis of the Diterpenoid Alkaloid Arcutinidine Using a Strategy Inspired by Chemical Network Analysis. J. Am. Chem. Soc. 2019.
Nie, W.; Gong, J.; Chen, Z.; Liu, J.; Tian, D.; Song, H.; Liu, X.-Y.; Qin, Y. Enantioselective Total Synthesis of (−)-Arcutinine. J. Am. Chem. Soc. 2019, 141 (24), 9712–9718.
Atta-ur-Rahman; Iqbal Choudhary, M.; Atta-ur-Rahman; Iqbal Choudhary, M.; Atta-ur-Rahman; Iqbal Choudhary, M. Diterpenoid and Steroidal Alkaloids. Nat. Prod. Rep. 1999, 16 (5), 619–635.
Wang, F.-P.; Chen, Q.-H.; Liu, X.-Y. Diterpenoid Alkaloids. Nat. Prod. Rep. 2010, 27 (4), 529.
Li, J.; Zhang, W.; Zhang, F.; Chen, Y.; Li, A. Total Synthesis of Longeracinphyllin A. J. Am. Chem. Soc. 2017, 139 (42), 14893–14896.
Zhou, S.; Guo, R.; Yang, P.; Li, A. Total Synthesis of Septedine and 7 Deoxyseptedine. J. Am. Chem. Soc. 2018, 140 (29), 9025–9029.
Ohnuma, T.; Oishi, T.; Ban, Y. Synthetic Entry to the Hexacyclic Aspidosperma Alkaloids. Total Synthesis of (±)-4-Hydroxyaspidofractinine. J. Chem. Soc., Chem. Commun. 1973, No. 9, 301–302.
Okamura, H.; Iwagawa, T.; Nakatani, M. A Base Catalyzed Diels-Alder Reaction of 3-Hydroxy-2-Pyrone. Tetrahedron Lett. 1995, 36 (33), 5939–5942.
Carlsen, P. H. J.; Katsuki, T.; Martin, V. S.; Sharpless, K. B. A Greatly Improved Procedure for Ruthenium Tetroxide Catalyzed Oxidations of Organic Compounds. J. Org. Chem. 1981, 46 (19), 3936–3938.
Hamlin, A. M.; Lapointe, D.; Owens, K.; Sarpong, R. Studies on C 20 -Diterpenoid Alkaloids: Synthesis of the Hetidine Framework and Its Application to the Synthesis of Dihydronavirine and the Atisine
Skeleton. J. Org. Chem. 2014, 79 (15), 6783–6800.
Marth, C. J.; Gallego, G. M.; Lee, J. C.; Lebold, T. P.; Kulyk, S.; Kou, K. G. M.; Qin, J.; Lilien, R.; Sarpong, R. Network-Analysis-Guided Synthesis of Weisaconitine D and Liljestrandinine. Nature 2015, 528 (7583), 493–498.
Доступные форматы для скачивания:
Скачать видео mp4
-
Информация по загрузке:



















